MICROWAVES
Microwaves are electromagnetic waves with wavelengths ranging from as long as one meter to as short as one millimetre, equivalent to frequencies between 300 MHz and 300 GHz. As the devices operating in this wavelength range have been more and more used, including therapeutic applications, the importance of the studies of their health effects increases constantly.
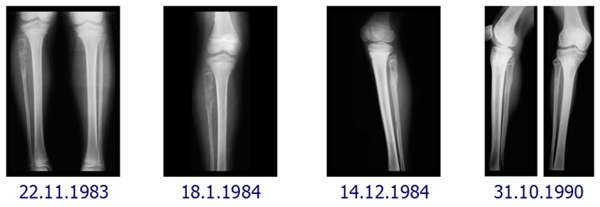
In the experiments on male Wistar rats irradiated with microwaves 53.57 MHz during 14 days, NSC 631570 was revealed to normalise the activity of aminotransferases ALT and AST as well as the serum concentration of the alpha-fetoprotein compared to the irradiated control group (233).
The effect of NSC 631570 (7 mg/kg intraperitoneal for 10 days) on the serum chemistry parameters of male Wistar rats during concomitant microwave irradiation (53.57 GHz, 10 mW/cm2, 20 min daily for 10 days) was studied. At the end of the study no significant changes were observed in the combined treated group compared to the control group. The authors concluded NSC 631570 can be used combined with microwave therapies (242).
TOXICOLOGIC STUDIES
On an induced hepatitis model the researchers studied whether NSC 631570 can protect liver cells from the toxic effect of acetaminophen overdosage. Indeed, NSC 631570 exerted a protective effect with the stimulation of liver macrophages (217).
In the tests on rats, the effects of chelidonine (50 or 100 mg/kg intraperitoneal) and NSC 631570 (7 or 14 mg/kg intraperitoneal) on some blood chemistry parameters after intoxication with copper chloride or lead chloride were studied. Both agents normalised the serum values of beta-2-microglobulin, creatinine, and urea. The effect of chelidonine was more pronounced (229, 234, 236). The treatment with NSC 631570 normalised also increased values of ALT and alpha-fetoprotein (193, 241).
In male Wistar rats with experimental acute ethylene glycol intoxication (3 or 3.5 g/kg intraperitoneal), the effect of NSC 631570 (7, 14 or 28 mg/kg intraperitoneal) on blood chemistry parameters was studied. Compared to the control group, the 10 day administration of NSC 631570 caused a significant decrease of serum urea concentration and increase of serum beta-2-microglobulin concentration (235, 257). In the study with lower ethylene glycol dose (2.5 g/kg), an increased serum concentration of beta-2-microglobulin was observed (240).
In male Wistar rats with experimental acute alcohol intoxication (methanol 4.5 g/kg intraperitoneal, ethanol 3 g/kg intraperitoneal, or ethylene glycol 3.5 g/kg intraperitoneal) the effect of NSC 631570 (28 mg/kg intraperitoneal as single dose or 10 day administration) on the aminotransferase activity (ALT and AST) and the serum alpha-fetoprotein concentration was studied. Compared to the control group, the single administration of NSC 631570 caused the drop of the increased AFP concentration (except the ethylene glycol group). The serum AFP concentration increased after the 10 day administration of NSC 631570. There were no changes in other parameters compared to the control (237, 248, 256, 257).
To study the effect of NSC 631570 on some chemistry parameters in the acute methanol intoxication, Wistar rats were treated with NSC 631570 for 10 days. The therapy alleviated the unfavourable consequences of the methanol intoxication, expressed by the normalization of the increased serum concentrations of beta-2-microglobulin and urea (192, 196, 227, 228).
NORMALISATION OF THE METABOLISM
Bone Metabolism and Osteoporosis
A series of animal tests was aimed to study the effect of NSC 631570 on bone density and mineral metabolism.
In a 6 month study on female ovariectomized rats, NSC 631570 has been revealed to inhibit the development of some indications of early osteoporosis (78). The blood parameters and transaminase activity in the NSC 631570 group did not differ from ones in the control group (79). Prolactin and progesterone concentrations were elevated, those of corticosterone and aldosterone diminished compared to the control ovariectomized group (57, 80).
The treatment of mature female rats with NSC 631570 at high dosage had no negative effect on the bone mineral density. A slight decrease of bone mineral content was observed (128).
After intermittent 3 month administration of NSC 631570 at high dosage to ovariectomized female rats the bone mineral density decreased (129).
The effect of NSC 631570 on the bone metabolism in rats was reviewed in an article. The author concluded this drug to affect especially the estrogen dependant mechanisms and has protective effect against osteoporosis (174).
In a set of animal tests, rat femur was used as a model for the study of the long-term effect of NSC 631570 on various bone parameters. NSC 631570 was revealed not to diminish the bone strength and density also at higher dosage (175, 176).
It was also studied how various doses of NSC 631570 affect the intensity of electron spin resonance (ESR) signal in intact and ovariectomized rats. The intensity of the signal correlates directly with the amount of free radicals in the tissue. The signal intensity was the lowest in the ovariectomized group with highest NSC 631570 dose and the highest in the intact group at the lowest dose of NSC 631570 (177).
In intact female rats the effect of NSC 631570 on the hormones involved into calcium metabolism was studied. In the highest dosage group the serum concentrations of corticosterone and progesterone decreased significantly and non-significantly, respectively. In the lowest dosage group the serum concentration of parathormone increased significantly. The serum values of calcitonin were not affected in either group (223).
The effect of NSC 631570 on the same hormones was also studied in female ovariectomized rats. In the middle dose group the corticosterone serum concentration dropped. There were no other changes in hormone values in all dose groups (224).
In the tests in male Wistar rats irradiated with 53.57 MHz microwaves for 14 days, NSC 631570 exerted practically no effect on the water content as well as organic and inorganic phases of pelvis bone compared to the irradiated control group (232).
The effects of NSC 631570 at different doses and/or strontium on the rat tooth intertubular dentine were analysed in cuts perpendicular to the dentinal tubes. The tooth surfaces and cross-section morphology and roughness were investigated with atomic force microscopy. The cross-section surface of intertubular dentine was analysed by roughness and fractal parameters. Histograms were prepared for the typical samples from all groups teeth analysed. Dentine cross section surfaces showed significant differences between the nano structures of normal rat teeth and those from animal treated with NSC 631570 and strontium (260).
THE EFFECT ON VARIOUS ENZYMES
In the tests on liver cells NSC 631570 was revealed to inhibit the alcohol dehydrogenase activity (178, 253).
The effect of NSC 631570 on the activity of trypsin-like enzymes was also studied (183, 222).
The effect of NSC 631570 on the concentration of the vasoactive intestinal peptide (VIP) was studied on the diabetes model in mice as well as on intact animals. NSC 631570 exerted no effect on the VIP concentration in intact mice. In diabetic mice the 10 day administration of NSC 631570 caused a significant increase of the VIP concentration in the lowest dose group (194, 225).
In the experiments on the rat liver the researchers revealed that NSC 631570, beside chelidonine, caused the strongest inhibition of the enzyme monoamine oxidase (MAO), indicating its antidepressant properties (197, 252).
In a review the researchers summarized the articles on the effect of NSC 631570 on the amino acid metabolism. They noted NSC 631570 exerts different effects on the amino acid pools in the body and in the malignant tissue (213).
In a set of experiments in rats the effects of NSC 631570 on various liver enzymes were studied. In a test, the effects of the NSC 631570 seven day administration on the lipid peroxidation and antioxidative function of the rat liver were explored. At daily dose of 2 mg/kg NSC 631570 the serum concentration of reduced glutathione decreased at 25% and the antioxidative function of the rat liver decreased at 49%. The glutathione reductase activity in the postmitochondrial fraction of the rat liver increased at 43% compared to the control group. There was no activation of the lipid peroxidation after the administration of 2 mg/kg NSC 631570, and the catalase as well as peroxidise activity did not differ from those in the control group. The authors suggested an assumption NSC 631570 to cause an oxidative stress in the tumor resulting in apoptosis (214).
The effects of NSC 631570 on the liver enzymes involved into the drug metabolism were studied. After the six day administration of NSC 631570 at a daily dose of 2 mg/kg, the activity of aminopyrine-N-demethylase increased by 35% and the one of glutathione-S-transferase by 55%. The concentrations of microsomal cytochromes P450 and b5 as well as the rate of ethylmorphine-N-demethylation were not affected (215).
INTERACTION WITH OTHER DRUGS
Non-Opioid Analgesics
In mice and rats the interaction of NSC 631570 and aminophenazone was studied. It was found that NSC 631570 affects the analgesic effect of aminophenazone in various ways. In the writhing syndrome test in mice as well as in tail-flick test in rats the antinoceptive effect of the analgesic was potentiated, whereas in the hot-plate test the analgesic effect of aminophenazone was reduced (20).
The analgesic effect of NSC 631570 was augmented by the nitric oxide synthase inhibitors in mice. These results suggest the endogenous nitric oxide can modify the analgesic effect of NSC 631570 (130).
Opioid Analgesics
The tests on mice and rats revealed NSC 631570 to modify the antinoceptive effect of opiates. In mice, for example, NSC 631570 potentiated the analgesic action of morphine in the hot plate test and in the tail flick test, however, reduced this effect in the writhing syndrome test (35).
In the studies in mice was revealed the 10 day intraperitoneal administration of NSC 631570 at high dose has an analgesic effect in mice. Combined administration of morphine and NSC 631570 reduced their antinoceptive effects reciprocally. The authors propose to avoid combined clinical use of these drugs (85, 86).
The analgesic action of NSC 631570 in mice was completely abolished by naltrexon, a pure opioid antagonist acting on all opioid receptors as a competitive antagonist (131).
Streptozotocin
The streptozotocin diabetes model was used in rats to explore how changes the liver and kidney function in these animals under the impact of NSC 631570. The 10 day administration of NSC 631570 to streptozotocin rats did not change the serum creatinine concentration, but increased the urea value. The authors concluded NSC 631570 should not be combined with streptozotocin (195, 226, 239).
Porphyrin Derivates
In the tests on murine sarcoma, mammary carcinoma, human colon carcinoma and melanoma cell lines NSC 631570 and porphyrin amino acid derivates to have synergetic action against cancer cell lines (53, 83).
Anticonvulsants
In Albino mice the interaction of NSC 631570 and various anticonvulsants such as diazepam, carbamazepine, diphenylhydantoin, phenobarbital, and valproate was studied. NSC 631570 potentiated the protective effect of valproate, whereas the effects of other anticonvulsants were not affected (34).
INTERACTION WITH OTHER THERAPY MODALITIES
Local hyperthermia
Hyperthermia is a type of treatment in which body tissue is exposed to high temperatures (up to 45°C), to damage and kill cancer cells, or to make cancer cells more sensitive to the effects of radiation and certain anticancer drugs. Local hyperthermia treatment (heat applied to a very small area, such as a tumor) is a well-established cancer treatment method with a simple basic principle: If a rise in temperature to 45°C can be obtained for one hour within a cancer tumor, the cancer cells will be destroyed. Primary malignant tumors have a bad blood circulation, which make them more sensitive to changes in temperature. The therapy with NSC 631570 has been successfully combined with local hyperthermia since many years (115, 144, 208).
In his speech at the 5th Vienna Dialog on the Holistic Medicine, Dr. B. Aschhoff reported on his experience in the NSC 631570 therapy and local hyperthermia. Summing up, following theses were noted: significant improvement of the quality of life; few adverse effects; NSC 631570 is safe and can be used in children also; wide indication range of the therapy (142).
Endovascular Laser Therapy / Photodynamic Therapy
The endovascular laser therapy as a part of photodynamic therapy (PDT) is a new method for systemic laser treatment and energy transfer to the human body. For therapeutic application red, infrared, green, and blue lasers are used. The photon stream applied intravenously leads to an improved microcirculation, activation of the immune system and mitochondria. Recently, NSC 631570 has been used as a sensitizer (Weber M, ‘The intravenous laser blood irradiation, a new therapeutic approach in immunology and cancer therapy”, presentation at the 2nd International Conference on Drug Discovery and Therapy, SL-341, Dubai, UAE, 2010).
NSC 631570 was administered to the patients with rheumatic diseases or high susceptibility to infections. In the lymphocytes from both patients group and from a control group, expression of IgG, proliferation marker Ki-67 and other marker molecules were estimated. There were no significant changes in the control group whereas in the NSC 631570 group the expression of IgG and Ki-67 increased significantly. Further increase of marker expression was achieved using additional endovascular low-level laser therapy. Circulating tumor cells from cancer patients were incubated with NSC 631570. Patients were treated with NSC 631570 alone or with NSC 631570 and endovascular laser. The tumor mass reduction was achieved in both groups but in the combined group this effect was more pronounced (Andrae F, ‘Ukrain based laser therapy in oncology’, presentation at the 2nd International Conference on Drug Discovery and Therapy, SL-274, Dubai, UAE, 2010).
Ozone
Empiric experience revealed NSC 631570 should not be combined with the ozone therapy. Generally, NSC 631570 should preferably be used as monotherapy. Other therapy modalities can be used in the breaks between the treatment cycles.
Others
In a pilot study it was elaborated how a method for determination of an optimal dosage for the therapy with NSC 631570 could be elaborated (the same for interferon-alpha, too). The method is based on the estimation of SS/SH groups ratio in serum (138).