- INTRODUCTION: THE FIRST MEDICAMENT THAT KILLS CANCER CELLS BUT NOT HEALTHY CELLS
- I. EFFICACY
- II. SAFETY
- III. QUALITY
- BIBLIOGRAPHY
UKRAIN (NSC 631570) is chelidonii radix special liquid extract. This is a complex produced from two approved substances – greater celandine alkaloids and thiotepa (2, 9, 145). Its quality proof is specified in German Pharmacopoea and Pharmacopoea Austriaca.
Ukrain is the first and only drug effective against cancer and more than 300 times less toxic than its sources substances (12, 39, 41, 59, 111, 141, 179). It has been proven in numerous in vitro, in vivo and clinical studies that this medicine in smaller dosage (5 mg) exerts immune modulating properties (2, 5, 8, 10, 14) whereas in larger dosage its effect is malignocytolytic (3, 6, 11). The therapeutic index of Ukrain is 1250 (therapeutic index is relation between toxic dose and the therapeutic one and reflects the safety of a drug). This is rather unusual for an anticancer drug and explains the good tolerability of NSC 631570. Therapeutic index of the common cytostatic drugs is in the range 1.4-1.8 and their overdosage can cause fatal consequences.
The phase I clinical study was performed on 19 healthy volunteers on the out-patient basis. Beside general clinical condition, following parameters were evaluated: blood count, clinical chemistry, immune values, electrolytes, microelements, neopterin. NSC 631570 was administered intramuscularly or intravenously daily, on the alternate days or every third day at a daily dose of from 5 up to 50 mg for 7-40 days. In a special case, the medicine was administered during three years at a total dose of 3500 mg divided into several therapy courses. No significant changes in clinical status were revealed at the examination. In the case of intramuscular administration, volunteers reported local pain, sometimes sleepiness, increased thirst and polyuria. In some cases, a light non-significant increase of the body temperature and minor blood pressure decrease were observed. The authors concluded NSC 631570 at single doses of 5, 10, 20 and 50 mg were well tolerated, also at prolonged administration. (37)
The Dose Finding Study (Phase II)
To find out the correct dosage for NSC 631570 a phase II clinical study was performed on 70 end stage cancer patients. The following parameters were estimated: physiologic values (heart beat rate, blood pressure, body temperature), blood count, clinical chemistry, electrolytes, immune values. The response on the therapy was evaluated by means of x-ray, ultrasonography and computed tomography (CT). NSC 631570 was administered intramuscularly or intravenously daily, on the alternate days, every third, every forth, or every fifth day. Single doses were 2.5, 5, 10, 15, 20, or 25 mg in ascending order (from 2.5 up to 25 mg), descending from 25 mg to 2.5 mg, or 5, 10, 15, 20 or 25 mg constantly. The duration of a therapy courses was 10-90 days. Breaks between courses varied from seven days up to three months. In all cases the therapy with NSC 631579 was well tolerated. In some patients the analgesics dosage could be reduced. The quality of life improved in the most cases. Subjective as well as objective symptoms and signs were observed like headache, dizziness, thirst, sweating, polyuria, fever (with the body temperature increase of 1-2 °C), and pain at the tumor and/or metastases area. Increased temperature at the tumor area was observed also. Temporary tumor swelling, increased heart beat rate and minor blood pressure decrease were observed as well. The intensity of such concomitants correlated with the response to the therapy. After full remission these concomitants were not anymore observed (21, 45).
In healthy volunteers, such concomitant events are observed not so extensively or not observed at all. It can be suggested they are triggered by the tumor degradation products. The intensity of these concomitants can be decreased with the detoxication measures.
According to the recent findings, to achieve the best results high doses of NSC 631570 should be used in turn with small ones. Higher doses destroy tumors and smaller doses helps to eliminate the tumor degradation products. This is why alternate doses of NSC 631570 are used, e.g. 5-20 mg, or 5-30 mg, 5-40 mg daily or on alternate days. NSC 631570 can be diluted with 5% dextrose. In the case of administering 20 g NSC 631570, higher doses of vitamin C (2-4 g) should precede the injection. There are reports on tumor responses after 10 day in-patient therapy courses with daily dose of 20 mg NSC 631570 intravenously. Due to its antiangiogenic effect NSC 631570 can bring about the tumor encapsulation improving its respectability.
The clinical efficacy of NSC 631570 has been proven by many researchers and is subject of numerous publications. More than 40 original articles keep records on the treatment of more than 750 patients, 332 of these were treated with NSC 631570 in controlled clinical trials. All researchers note the efficacy and safety of NSC 631570.
Ukrain can cause the full regression of the main tumour and also of metastases. In the treatment of advanced tumours Ukrain can improve the quality of life and prolong survival. Many clinical studies have proved this, such as those of the work groups led by Prof. Beger in Germany and of Prof. Zemskov in Ukraine with pancreatic cancer (182, 186, 187, 205, 247; 154, 185), as well as groups led by Prof. Susak and Prof. Bondar in Ukraine with colon cancer (67, 106, 108, 112). Neoadjuvant (before surgery) use of Ukrain can induce encapsulation of tumours as revealed the studies by the researchers of Grodno Medical University (Grodno, Belarus) in breast cancer (68-73, 114, 157-159).
In an open study total 203 advanced cancer patients were treated with NSC 631570 and partially with local hyperthermia (37.4%) after all conventional treatment modalities had failed and the disease progressed or relapsed. Full remission was achieved in 41 cases (20.2%), partial remission – in 122 cases (60.1%). Seminoma and prostate cancer responded especially well with remission rate of more than 75% (144, 161).
In a controlled randomised study by Prof. Beger et al. in the Ulm University Hospital, Germany, the therapy with NSC 631570 and gemcitabine doubled the survival rate in the patients with inoperable advanced pancreatic cancer (182). The longest survival was 19 months in the group treated with gemcitabine alone, 26 months in the combined group, and in the NSC 631570 alone group two patients were alive after 28 months. NSC 631570 was well tolerated. The study authors consider further evaluation of NSC 631570 as justified whereas the quality of life of the patients improved (186).
Ukrain in pancreas cancer: palliative therapy
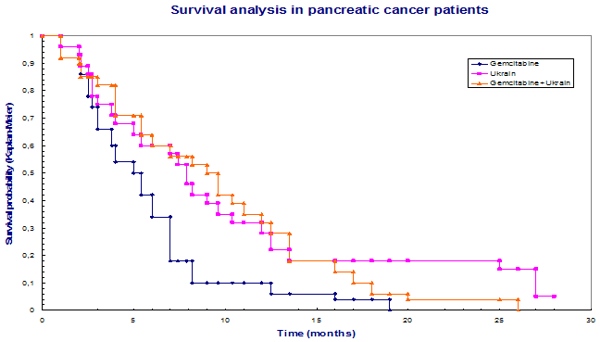
Gansauge, Beger et al: Langenbeck¢s Archives of Surgery, 2002
Patients were further observed after the conclusion of the study and it was noted that UKRAIN was well tolerated and could be administered without problem to all patients. UKRAIN brought about a significant increase in survival time in comparison to therapy with gemcitabine alone. Combination therapy with gemcitabine and UKRAIN showed no advantage over monotherapy with UKRAIN. The longest survival in the gemcitabine group was 19 months, 21 months in the gemcitabine+Ukrain group, and in the Ukrain group a patient was still alive after 28 months. The authors concluded: ‘As a result of this study we highly recommend the treatment of patients suffering from advanced pancreatic cancer with Ukrain’ (187).
2007 the results of another clinical study by the same research team were published. This time the efficacy of the adjuvant therapy with NSC 631570 has been demonstrated in the patients with advanced pancreatic cancer after surgery. The patients were treated with a combination of NSC 631570 and gemcitabine.
The median survival was 33.8 months and the 5-year survival rate was 23.3% which is clearly better than results reported in the earlier studies without NSC 631570, with the median survival of 20.1 months and the 5-year survival rate was 21% (http://content.nejm.org/cgi/content/ abstract/350/12/1200 ). Moreover, NSC 631570 at therapeutic dose range has only minimal adverse effects, improves the quality of life of patients and can be administered also on out-patient basis. All these features distinguishes this drug favourable compared to the standard cytostatic agents.
Adjuvant therapy in pancreas cancer: comparison of three studies
|
Author |
Neoptolemos |
Kurosaki |
Gansauge |
|
Year |
2001 |
2004 |
2007 |
|
Number of patients |
238 |
16 |
30 |
|
Therapy |
5-FU/FS |
Gemcitabine |
NSC 631570 / Gemcitabine |
|
Relapse free survival |
k. A. |
16,8 Mo. |
26 Mo. |
|
Median survival |
19,7 Mo. |
20,4 Mo. |
37,6 Mo. |
Again, this publication supports the efficacy (and safety) of the use of Ukrain as it demonstrates a considerable prolongation of survival compared to what is known from other clinical studies (247).
Other researcher confirmed the efficacy of NSC 631570 in pancreatic carcinoma (205, 208, 209), while the partial remission rate was as high as 85.7% in one study (207). The longest survival in palliative therapy was more than six years (185, 186).
Histological changes caused by the treatment with NSC 631570 in the pancreatic tumor and in the surrounding tissue were profoundly studied. NSC 631570 has been revealed to bring about the fibrotic and sclerotic transformation of the tumour. Perivascular sclerosis has also occurred (206).
In a controlled randomized clinical study by the National Medical University (Kyiv, Ukraine) colon cancer patients were treated with NSC 631570 or with 5-fuorouracil and x-ray therapy. The survival rate after 21 months was 78.6% in the NSC 631570 group and 33.3% in the group treated with 5-FU and radiotherapy (67).
Within a randomized study in the Doneck Regional Cancer Center (Ukraine) rectal cancer patients received either high-dose radiotherapy and 5-FU before surgery, or the therapy with NSC 631570: one course before surgery (10 mg every second day up to 60 mg) and another course afterwards (up to 40 mg). During following 14 months, relapses occurred in six patients (25%) from the combined group and in 2 patients (8.3%) in the NSC 631570 group. Two year relapse rate was 33.3% (8 patients) in the combined group and 16.7% (4 patients) in the NSC 631570 group (112). Now, 11 years after this publication 18 from 24 patients (75%) in the NSC 631570 group are still alive.
The efficacy of NSC 631570 in prostate cancer has been confirmed in a controlled clinical study. In the study patients, all standard treatment modalities had been exhausted. The cancer relapsed and/or progressed and no therapy protocol was available. The patients were treated with NSC 631570 and partially with local hyperthermia. Following results were achieved: full remission in 54 patients (73%), partial remission in 16 patients (22%). Only in 4 patients (5%) the therapy did not affect the course of the disease (201).
|
Total number of patients |
Full remission |
Partial remission |
Disease progression |
|
74 |
54 |
16 |
4 |
|
100% |
73% |
22% |
5% |
The good efficacy of NSC 631570 in prostate cancer has been confirmed in another study (155).
In a controlled clinical study conducted at the University Grodno (Grodno, Belarus), after the therapy with NSC 631570 the hardening of the tumor, a slight increase in the tumor size (5-10%) and proliferation of connective tissues were observed. The T4/T8 lymphocytes ratio increased by 30%. The tumours appeared harder and slightly enlarged after NSC 631570 therapy, and were easier to detect by ultrasound or radiological examination. Metastatic lymph nodes were also hardened and sclerotic (fibrous). Tumours and metastatic lymph nodes were clearly demarcated from healthy tissue and therefore easier to remove. Complications such as prolonged lymphorrhoea (leakage of lymph onto the skin surface), skin necrosis (death of skin tissue), suppuration of the wound, and pneumonia, all occurred in patients from the two NSC 631570 groups at only half the rate that they appeared in patients from the control group. Based on the results of this study the scientists from Grodno recommended the use of NSC 631570, at the higher dosage, in all breast cancer operations (54, 68-70, 114). Other parameters were also evaluated, e.g. hormones (T3, T4, cortisol, progesterone, estradiol, prolactin; 71), immune values (lymphocytes, immune globulins, complement, phagocytic activity; 72), morphologic and cytochemical changes (73, 110), amino acids and their derivates in plasma (74, 109) and in the tumor tissue (75).
In a series of articles the researchers have studied the effect of NSC 631570 on various parameters in breast cancer patients (157-160). Best results were achieved with higher dosage of NSC 631570. Almost every patient noted the improvement of the general well-being, sleep and appetite. During the surgery, the tumors as well as involved lymph nodes were presented sclerotic and well demarcated from the surrounding tissue. This alleviated the surgical removal of the tumor considerably (158). In the tumor tissue, increased concentration of the amino acid proline was revealed indicating augmented production of connective tissue that demarcates the tumor from surrounding tissue (159). NSC 631570 improved also the amino acid balance of patients (160).
In a study NSC 631570 caused full remission in three patients for six months (113, 137).
Biochemical evaluation revealed NSC 631570 had favourably affected the amino acid metabolism (156).
The first publication on the using NSC 631570 in malignant melanoma describes the full remission in a patient with metastases to the lung (91).
A long lasting remission (more than 10 years without recurrence) has been observed in a patient with malignant nodular melanoma after the treatment with NSC 631570. At the beginning of the NSC 631570 therapy liver metastases were present and melanin was excreted with urine (92).
The effects of NSC 631570 alone and in combination with the pathogen associated molecules (PAM) on the cell cycle and apoptotic induction were compared in two melanoma cell lines MM-4 and MM-4M2 with different metastatic properties (cell division rate, hematogenous metastazing, sensitivity to the TNF-induced apoptosis).
Apoptosis induction and cell viability were analyzed using trypan blue exclusion test, morphological criteria, DNA gel electrophoresis, and flow cytometry. Cell cycle distribution of tumor cells was estimated by flow cytometry. The therapy with NSC 631570 induced apoptosis in both melanoma cell lines in a dose-dependant matter. The cell line with higher metastatic potential was more sensitive to NSC 631570. In the cell line with low metastatic potential, combined use of NSC 631570 and PAM induced apoptosis more effectively (261).
NSC 631570 has been successfully used in the treatment of brain tumors (101, 102).
In a review on the clinical studies with NSC 631570 performed so far the researchers from the Universities of Exeter & Plymouth suggested this agent to have potential as an anticancer drug (238).
Earlier, there was a report on the successful using NSC 631570 in the treatment of ovarian cancer (97). Also in the tests of National Cancer Institute (Bethesda, Maryland, USA) NSC 631570 was toxic against all ovarian cancer cell lines tested (190). Other authors reported on good results in the therapy of cervical cancer (27, 96).
Ukrain (NSC 631570): autofluorescence under UV-light
In ultraviolet light Ukrain fluoresces in the yellowish green range of spectrum. Excitation frequencies are within a range of 220 to 490 nm. The spectral width of the fluorescence extends from 410 to 665 nm (4).
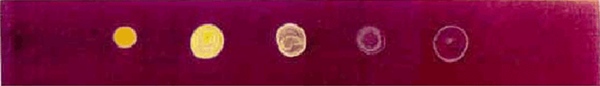
Thin-layer chromatography plate with drops of Ukrain under UV light. On the left, Ukrain in a concentration of 10 mg/ml in distilled water. Then serial dilutions by a factor of 10 each time (1 mg/ml; 0.1 mg/ml,…).

Patient N.E., aged 82. 1½ year history of multiple and exulcerating basaliomas at the cheek-nose area.

The same patient under UV-light at 254 nm three minutes after the first intramuscular injection of 5 ml Ukrain. Strong fluorescence of the tumours and surrounding tissue is visible. Ukrain administered after one week produced only slight fluorescence. A considerable regression of the tumours was also observed.
The selective accumulation of NSC 631570 in the tumor tissue proven due to autofluorescence
The selective effect of NSC 631570 on cancer cells has been confirmed due to its autofluorescence under UV light (4). First time this feature of NSC 631570 was presented 1983 at 13th International Congress of Chemotherapy in Vienna. It has been confirmed in this work NSC 631570 to selectively accumulate in cancer cells. The accumulation of NSC 631570 in cancer cells correlates with the efficacy of the drug. With the elimination of the preparation from the body the intensity of the fluorescence decreases also (1).
At this congress, the first reports on the successful using NSC 631570 in the treatment of the end-stage cancer patients were presented, where all standard therapy modalities had failed. Though poor prognosis, NSC 631570 brought about full remission in a part of the patients and some of those are still alive.
